Solid Selenium Dioxide With Excess Aqueous Potassium Hydroxide
In its aqueous form its appearance is that of a clear solution. Sodium hydroxide and potassium hydroxide as solids absorb moisture and carbon dioxide from the air to form the bicarbonates.
Along with sodium hydroxide KOH is a prototypical strong base.
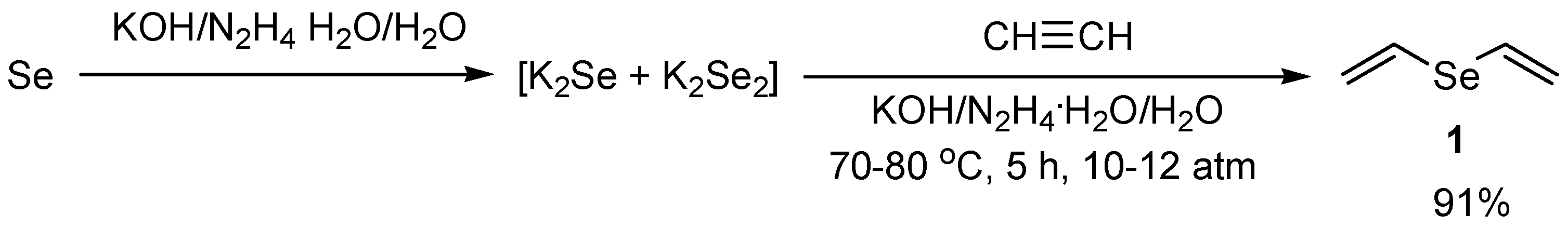
. . The equation for the reaction is shown. Potassium hydroxide -Formula.
The added advantage of the process is the accessibility of internal passages for coatings removal. Potassium hydroxide is also known as caustic potash lye and potash lye. Exposure to moist air or water.
Product Identifiers -Product Name. POTASSIUM HYDROXIDE 5 CONC. We need 1 potassium ion to balance one hydroxide ion making the formula KOH.
SeO₂ 2KOH K₂SeO₃ H₂O. Solid oxygen 00013 219 183 very poor sulfur 21 115 445 poor selenium 221 685 poor tellurium 62 450 988 quite good polonium 92 254 962 good a i Use the information in the table to suggest the density of selenium at room temperature. Solid SeO 2 is a one-dimensional polymer the chain consisting of alternating selenium and oxygen atoms.
250 cm3 of aqueous potassium hydroxide A student titrated 250 cm3 of 00500 mol dm 3 aqueous potassium hydroxide with dilute sulfuric acid in the presence of an indicator. Potassium hydroxide is an alkali metal having the chemical formula KOH. Therefore it has many industrial and laboratory applications.
Potassium hydroxide is an inorganic compound which is denoted by the chemical formula KOH. Aqueous solutions also absorb carbon dioxide to form bicarbonate. It has many industrial and niche applications most of which exploit its caustic nature and its reactivity toward acids.
Potassium hydroxide is an inorganic compound with the formula KOH and is commonly called caustic potash. It should be stored in a tight container when not in use and should not be 43. 6 CLE 2018 062043N18 4 aDilute sulfuric acid and aqueous potassium hydroxide can be used to make potassium sulfate crystals using a method that includes titration.
Potassium hydroxide KOH or HKO CID 14797 - structure chemical names physical and chemical properties classification patents literature biological activities. They are potassium sulfate K 2 SO. Conical flask burette filled with sulfuric acid 250 cm 3 of potassium hydroxide solution a 250 cm 3 of potassium hydroxide concentration 253 mol dm 3 was neutralised by 282 cm 3 of dilute sulfuric acid.
In its solid form KOH can exist as white to slightly yellow. Potassium 42 hydroxide is highly corrosive. Digestion in molten potassium hydroxide melting point 361C 682F has been found to accelerate removal of YSZ coatings Warnes and Schilbe 2001 compared with aqueous alkali solution in an autoclaveRemoval times are reduced from hours to several minutes.
They are both made by titration. An estimated 700000 to 800000 tonnes were produced in 2005. The bridging Se-O bond lengths are 179 pm and the terminal Se-O distance is 162 pm.
How many grams of the precipitate are formed if 150 grams of aq potassium hydroxide are used as a reactant. 41 air potassium hydroxide rapidly absorbs moisture and carbon dioxide and deliquesces. Handled without protective gear.
More concentrated the solution the faster it absorbs. The relative stereochemistry at Se alternates along the polymer chain syndiotacticIn the gas phase. The molar mass of potassium hydroxide is 5611 gmol.
What is Potassium Hydroxide. Conical flask dilute sulfuric acid 250 cm3 of aqueous potassium hydroxide A student titrated 250cm3 of 00500 mol dm 3 aqueous potassium hydroxide with dilute sulfuric acid in the presence of an indicator. Solutions stored in flasks with ground glass stoppers may leak air and freeze the stoppers preventing removal.
The balanced chemical equation for the reaction between selenium dioxide with aqueous potassium hydroxide. Write balanced equations for the following reactions. Answer 1 of 2.
Hence the balanced chemical equations for the reactions are a K₂O H₂O 2KOH. This alkali metal hydroxide is a very powerful base. KOH -Molecular Mass.
A Potassium metal. Selenium dioxide SeO2 or O2Se CID 24007 - structure chemical names physical and chemical properties classification patents literature biological activities. A potassium oxide with water b diphosphorus trioxide with water c chromiumIII oxide with dilute hydrochloric acid d selenium dioxide with aqueous potassium hydroxide.
Electrolyte is a concentrated aqueous solution of potassium hydroxide sodium hydroxide and lithium hydroxide 59 mol L 1. At room temperature it is a colorless solid and is a strong base. An aqueous solution of potassium hydroxide is mixed with an aq of magnesium chloride and you get a clumpy white precipitate that forms.
Depending on the application electrolyte composition is the result of a combination of high rate low and high temperature and cycle life requirements. The physical and chemical properties of potassium hydroxide are summarized in Table 1. SeO2 s 2KOHaq K2SeO3aq H2Ol SeO2s2KOHaqK2SeO3aqH2Ol Home.
PRODUCT AND COMPANY IDENTIFICATION 11. K2O H2O 2KOH. Potassium Hydroxide Revision Date 24-Dec-2021 Reactive Hazard Yes Stability Moisture sensitive.
To balance the equation we place a coefficient of 2 in front of the potassium hydroxide. POTASSIUM HYDROXIDE CONC 5. The potassium has a charge of K and hydroxide has a charge of OH.
Write a complete and balanced equation to calculate the stoichiometry question. The reaction between selenium dioxide with aqueous potassium hydroxide gives potassium selenite and water. H 2 SO 4 2KOH K 2 SO 4.
K2O H2O KOH. The common name of potassium hydroxide is caustic potash. How much carbon dioxide escapes as a gas and how much reacts with aqueous potassium hydroxide depends on several factors.
Incompatible Materials Water Metals Acids Hazardous Decomposition ProductsPotassium oxides. E Wri te a balanced equation for the reaction of aqueous potassium hydroxide with aqueous iron III chloride to form solid iron Ill hydroxide. Potassium hydroxide is an ionic compound.
What is the product of selenium dioxide plus aqueous potassium hydroxide. 5611 gmol -REACH Registration Number. 773 Write a balanced equation for the reaction that occurs in each of the following cases.
Rate of CO_2 being bubbled through this solution. Concentration of KOH in the water. Each Se atom is pyramidal and bears a terminal oxide group.
B solid barium sulfide with trioxygen c solid barium dioxide 2- and water d potassium hydroxide solution with carbon dioxide e sodium sulfide solution with dilute sulfuric acid f sodium sulfite solution and sulfuric acid g sodium sulfite solution with cyclo-octasulfur. A Ruls COgRuCOsAg b CaHsNO202 CO2H20NO c NH NO N202H2O d Write a balanced equation for the reaction of solid selenium Sea with fluorine gas to produce gaseous selenium hexafluoride. KOH is noteworthy as.
The volume of dilute sulfuric acid needed to neutralise the aqueous potassium hydroxide was 200 cm3. Conditions to Avoid Avoid dust formation.
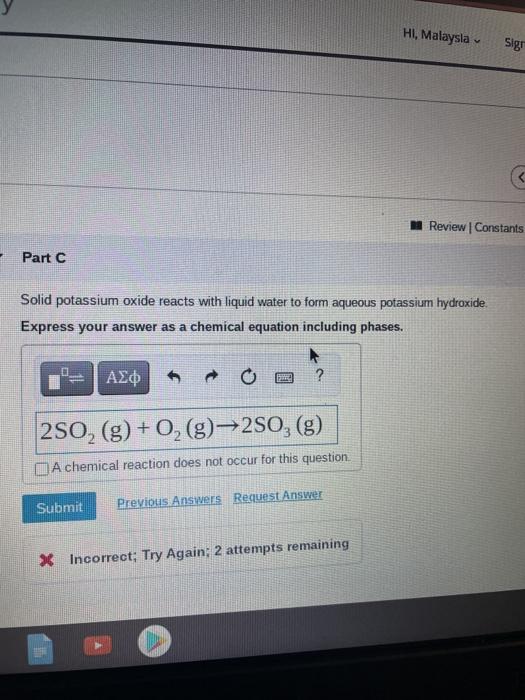
Solved Hi Malaysia Sigi Review Constants Part C Solid Chegg Com

Www Xtremepapers Com University Of Cambridge International Examinations General Certificate Of Education Ordinary Level 5070 21

Solved Write Balanced Equations For The Following Reactions A Potassium Oxide With Water B Diphosphorus Trioxide With Water C Chromium Iii Oxide With Dilute Hydrochloric Acid D Selenium Dioxide With Aqueous Potassium Hydroxide

Solved Write Balanced Equations For The Following Reactions Chegg Com

Chemistry 134 Problem Set Introduction
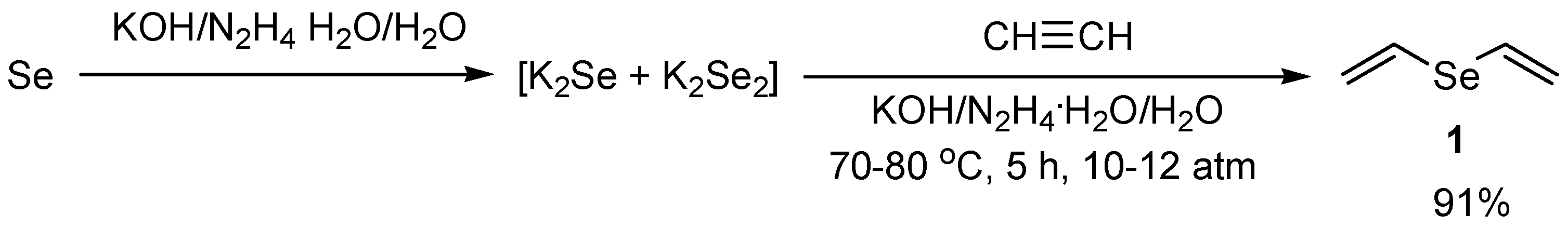
Molecules Free Full Text Selenium Dihalides Click Chemistry Highly Efficient Stereoselective Addition To Alkynes And Evaluation Of Glutathione Peroxidase Like Activity Of Bis E 2 Halovinyl Selenides Html
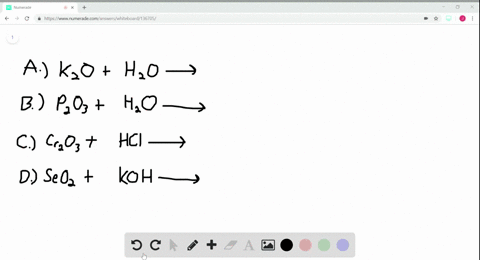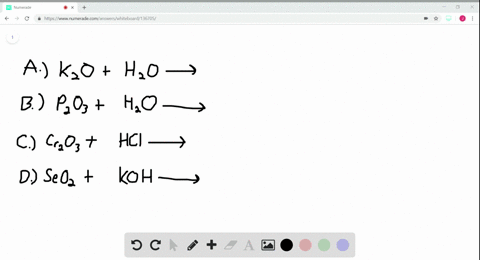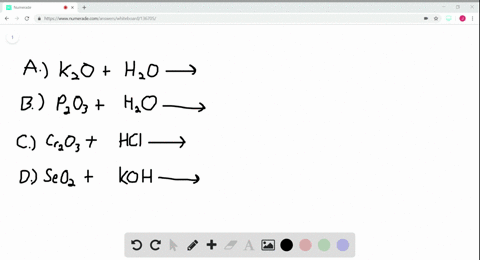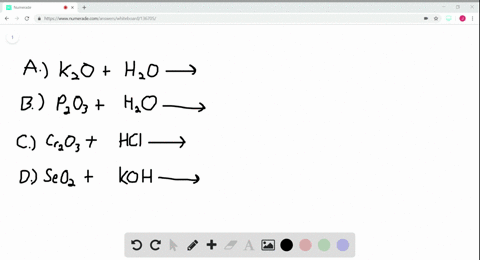
Solved Write Balanced Equations For The Following Reactions A Potassium Oxide With Water B Diphosphorus Trioxide With Water C Chromium Iii Oxide With Dilute Hydrochloric Acid D Selenium Dioxide With Aqueous Potassium Hydroxide
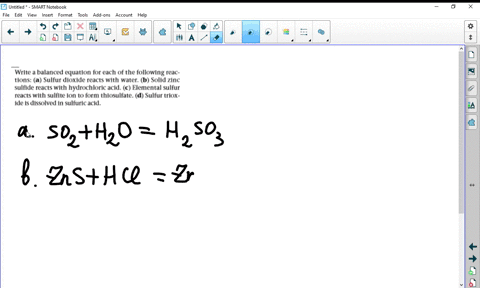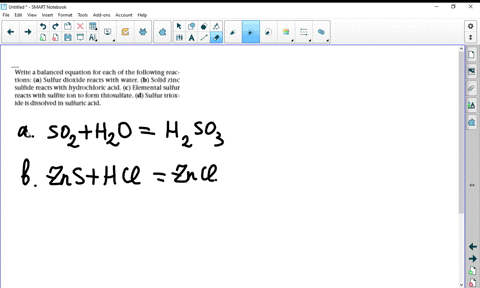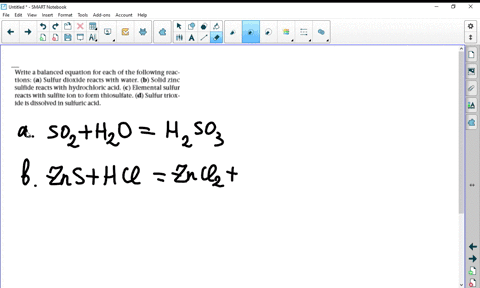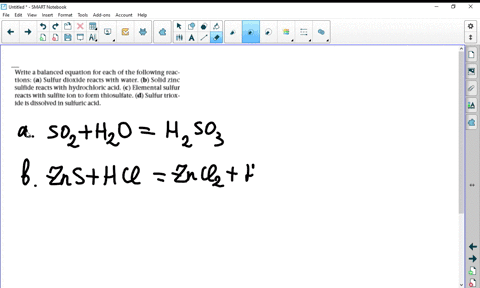
Solved Write Balanced Equations For The Following Reactions A Potassium Oxide With Water B Diphosphorus Trioxide With Water C Chromium Iii Oxide With Dilute Hydrochloric Acid D Selenium Dioxide With Aqueous Potassium Hydroxide









Comments
Post a Comment